Results for 'DEMA mission'

Leading From the Front: 3 Leadership Lessons From Mark Cuban
Dec 17th • 6 mins read

Leadership Lab: 5 Ways Biopharma Execs Can Restore Trust, Retain Talent After Layoffs
Jun 25th • 4 mins read

Leadership Lab: How To Spot When Employees Are About To Walk Away
Oct 22nd • 7 mins read

Leadership Lab: 4 Ways Biopharma Leaders Can Prepare for Media Interviews
Aug 20th • 6 mins read

New Book Unites Oncology’s Brightest Minds To Innovate Cancer Cures
Sep 9th • 5 mins read


MSL Hiring and Recruitment: 5 Ways to Support Diversity and Inclusion
May 19th • 2 mins read

Presentation Nails and Fails: 7 Tips to Ace Your Next MSL Presentation
Oct 12th • 1 min read



Comparative study on anticancer drug access times between FDA, EMA and the French temporary authorisation for use program over 13 years
Apr 7th • 12 mins read

Does biomarker use in oncology improve clinical trial failure risk? A large-scale analysis
Feb 23rd • 8 mins read

Assessment of Food and Drug Administration- and European Medicines Agency-Approved Systemic Oncology Therapies and Clinically Meaningful Improvements in Quality of Life: A Systematic Review
Feb 11th • 4 mins read
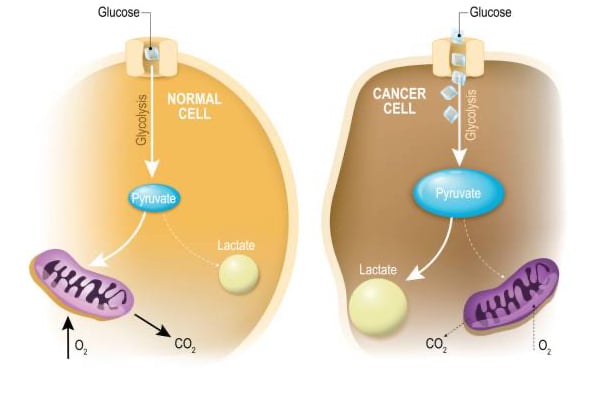
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read

Safeguarding cancer research funding by European charities amidst the COVID-19 pandemic
Nov 22nd • 3 mins read
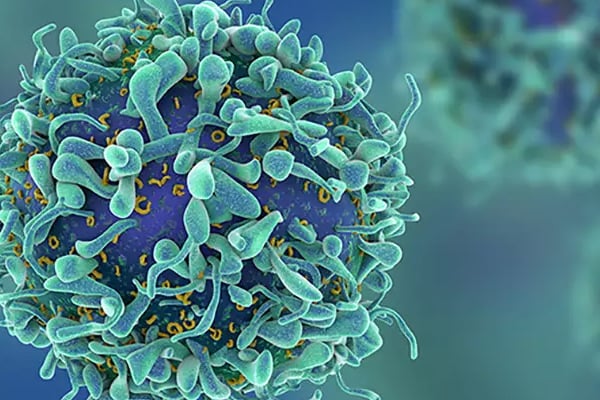
Publication statuses of clinical trials supporting FDA-approved immune checkpoint inhibitors: a meta-epidemiological investigation
Oct 24th • 18 mins read

Level of evidence used in recommendations by the National Comprehensive Cancer Network (NCCN) guidelines beyond Food and Drug Administration approvals
Aug 2nd • 8 mins read

A Comprehensive Comparison of Additional Benefit Assessment Methods Applied by Institute for Quality and Efficiency in Health Care and European Society for Medical Oncology for Time-to-Event Endpoints After Significant Phase III Trials—A Simulation Study
Jun 28th • 30 mins read

Patient involvement: A must-have in medicine development, but is it being overlooked in a cost-constrained environment?
May 9th • 5 mins read

Value assessment of NMPA-approved new cancer drugs for solid cancer in China, 2016-2020
Feb 24th • 8 mins read
